Successful Sample Preparation for Scanning Electron Microscopy (SEM): A Guide – AZoM
We use cookies to enhance your experience. By continuing to browse this site you agree to our use of cookies. More info.
Scanning electron microscopes use beams of electrons rather than beams of light, allowing us to see below the diffraction limit of conventional optical microscopes and image structures on the nanoscale.
However, an important caveat of electron microscopy is that samples must be exposed to harsh conditions.
Though light is transmitted easily through air, electrons are not. So, in order to image a sample using Scanning Electron Microscopy (SEM), it must be exposed to a hard vacuum.
Additionally, it can be complex or even impossible to image non-conductive samples without first coating them in a conductive layer, given that imaging relies on electron transfer between microscope and sample.
Though SEM sample preparation can be complicated, it is vital that it is performed correctly and carefully to create meaningful and detailed images. This guide provides a practical outline of SEM sample preparation, ranging from sample collection up to the point of imaging.
The initial step in SEM imaging, as indeed it is with all types of microscopy, is for sample collection to take place.
There are a number of typical rules which apply here. Of course, it is necessary to ensure that the sample chosen represents the surface, bulk material or population in question.
Taking photographs both before and after sample collection is encouraged to ensure that there is a record of what the sample looks like in its natural state.
Another important facet of sample collection concerns the containers used for transport and sample storage; they must be selected and handled in a way that keeps the sample protected and from damage.
Sample preparation is termed a “clean” process, which simply means that at every stage, from sample collection onwards, gloves should be worn.
Two of the main determining factors in SEM sample preparation are the moisture content of the samples and their rigidity.
It may be unsurprising that these elements are closely linked: for example, hard samples are often dry (e.g., composite materials or nanowire samples),1,2 while soft samples tend to have a high moisture content (e.g., cell cultures or biological samples).3
Soft matter samples, like living cells small organisms, and tissues, require fixation and dehydration prior to imaging. Conversely, dry, hard samples don’t require a lot of treatment and are relatively simple to image.
Fixation is the term given to the chemical treatment of samples performed to preserve and stabilize their structure.
Fixation typically takes place by use of incubation in a buffered chemical fixative, like glutaraldehyde, formaldehyde or tannic acid; and subsequently is often followed by post-fixation using osmium tetroxide.4 Bypassing this fixation process will naturally lead to sample damage during the dehydration phase.
Samples can be rinsed and dehydrated after fixation. If a sample contains any moisture, it is necessary to perform dehydration: when exposed to the high vacuum conditions inside the microscope, all samples must be free of water content.5
Though dehydration using air-drying or chemicals is fast, it does not necessarily offer the best results. In fact, techniques like these can impact the sample’s integrity and risk damage, particularly in the case of chemical-led rapid dehydration that uses chemicals like HMDS (hexamethyldisilazane).
Both Critical point drying (CPD) or freeze-drying are typically used to achieve high-quality results – both named processes prevent sample damage by bypassing an abrupt liquid-to-gas phase transition.
Critical point drying (also termed supercritical drying) is the name given to a high-temperature, high-pressure process that allows a liquid to transition smoothly into a gas without passing a sudden phase boundary.6
The surface tension between liquid and gaseous state, at the critical point, is zero – thus liquid can be removed from the samples without damaging them.
Water is typically first replaced with ethanol or acetone (by soaking the sample in series of increasing concentrations of those solvents) before putting it into a CPD machine as it has a high temperature at its critical point and it is not easily miscible with liquid CO2.
It is relatively easy to dry samples and preserve their natural appearance as CO2 has a low temperature at the critical point (31.0 °C).
Freeze-drying is a less complex process. Freeze-drying is simply accomplished by placing the given sample into a freeze-drying machine. Inside this machine, water is first frozen and subsequently sublimated from the solid phase into the gas phase.
Different results are produced by freeze-drying and CPD. Though CPD is considered effective for samples imaged at up to around 5000x magnification, visible structural damage is typically visible beyond this magnification.
Given that liquid CO2 is a powerful solvent, some samples can also be partially dissolved by the chemicals involved in CPD. This, therefore, prohibits CPD from being used, which is highly soluble in CO2.
However, it is important to note that freeze-drying does not lead to the dissolution of the sample and generally enables the use of higher magnifications. Of course, typically, freeze-drying produces molecular artifacts like collapse and aggregation, and soluble elements can be relocated within the sample.
It is essential that samples are moved to dry storage immediately after drying to prevent the accumulation of environmental moisture.
When it comes to revealing the desired surface for imaging, there are three broad classes of the method. These are as follows:
Very smooth surfaces are required for imaging modalities like Energy-Dispersive X-ray Spectroscopy (EDS) – which means polishing is crucial.
Conversely, polishing is not necessary for ordinary SEM, which is designed to probe morphology. The level of magnification used, the type of features being imaged and the properties of the sample itself determine which of these techniques are used.
A nitrogen blower or an air blower can be a productive way of removing sample dust. It is not recommended that “canned air” or “air dusters” are used for SEM preparation. Such products can risk damaging the sample as they often contain other chemicals.
It is key to ensure that electrically conductive contact is made between the stub and the top of the sample when mounting a sample. Thus, as shown in the diagram below, when using an adhesive agent, there must be enough used to enable a continuous layer of coating to bridge between the sample and the stub.

Image Credit: Quorum Technologies Ltd
Though carbon tabs and carbon tape provide utility for up to 100,000x magnification, they run the risk of producing outgassing or creeping within the SEM environment. For these reasons, their use is not recommended for higher magnifications. Taking this precaution ensures that charge accumulation on the top of the sample is prevented.
Generally, for powders, it is sufficient to apply a small amount of material to a piece of carbon tape using a single-use brush. One brush is typically enough, and the amount of material deposited on the tape can be checked using an optical microscope.
Nanoparticles should first be dissolved using an appropriate solvent (e.g., ethanol or isopropanol). It is recommended that you mount a silicon chip to a stub using carbon cement and then clean it thoroughly. A 3-5 microliter droplet of a solution is required to give good sample dispersion.
Finally, the last stage before images can be captured in an SEM is coating. Often, modern microscope manufacturers claim that coating is not needed for SEM imaging thanks to modern microscopes’ charge compensation mechanisms and low voltages. However, this advice should be met with scrutiny.
Charging reduces image quality even at low voltages, despite the fact that charging is less of an issue in modern microscopes than in older models. For instance, the image below shows extreme charging artefacts even though it was captured at only 1 keV.
Image Credit: Quorum Technologies Ltd
To ensure that no charge can “escape” the sample (instead of accumulating and causing image distortions), coating is essential. However, this is just one of many advantages that coating offers.
The signal can be localized to the very surface of the sample thanks to a metallic coating. This gives more detailed information about the surface, whilst improving the signal-to-noise ratio, secondary electron emission and image contrast.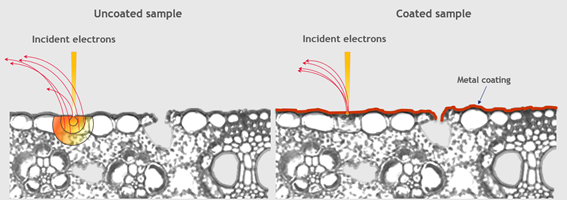
Image Credit: Quorum Technologies Ltd
Additionally, the risk of contamination can be lessened by coating. Contamination has two faces in SEM. Firstly, it can reduce image quality, and secondly, it can also escape the sample and result in damaging the microscope itself.
Immediate coating of the sample minimizes any risk of microscope contamination, as it effectively “seals” the sample at the same time as improving image quality.
Another effective use of coating is that it is effective at reducing beam damage, thanks to its localized heat. In this instance, sustained viewing is enabled in one location because the thermal conductivity of metallic coatings ensures that heat can flow away from the target site.
Naturally, this offers particular utility in regard to sensitive biological samples which risk being damaged or evaporated by intense heat.
When it comes to SEM coating, there are many different suitable materials to choose from, which often depend on the desired magnification. When coated under the same conditions, different metals have different grain sizes – and when imaging small features, only small grain sizes are suitable.
Iridium, tungsten or platinum are key for taking images of nanometer-sized objects. Chromium, gold and silver are also suitable for smaller magnifications.
Chromium, palladium and carbon are best suited for Backscattered Electron (BSE) imaging. If EDS is preferable, carbon is the obvious choice, but tungsten and gold are also good options. The key step here is coating with a material that gives peaks that are distant from the elements a study is examining.
When imaging using SEM, Quorum understands that proper sample preparation makes all the difference. Quorum’s Q Plus Series of coaters provides a world-class standard of coating but avoids the hefty cost of big-name manufacturers.
Both oxidizing and non-oxidizing metals are possible with Quorum’s turbomolecular-pumped coaters, whilst their rotary-pumped low-cost sputter coaters are suitable for non-oxidizing metals.
Sputter coating and evaporating carbon coating for SEM, FE-SEM and TEM applications are possible with the Q Plus Series.
This information has been sourced, reviewed and adapted from materials provided by Quorum Technologies Ltd.
For more information on this source, please visit Quorum Technologies Ltd.
Please use one of the following formats to cite this article in your essay, paper or report:
APA
Quorum Technologies Ltd. (2021, September 28). Successful Sample Preparation for Scanning Electron Microscopy (SEM): A Guide. AZoM. Retrieved on January 15, 2022 from https://www.azom.com/article.aspx?ArticleID=20764.
MLA
Quorum Technologies Ltd. "Successful Sample Preparation for Scanning Electron Microscopy (SEM): A Guide". AZoM. 15 January 2022. <https://www.azom.com/article.aspx?ArticleID=20764>.
Chicago
Quorum Technologies Ltd. "Successful Sample Preparation for Scanning Electron Microscopy (SEM): A Guide". AZoM. https://www.azom.com/article.aspx?ArticleID=20764. (accessed January 15, 2022).
Harvard
Quorum Technologies Ltd. 2021. Successful Sample Preparation for Scanning Electron Microscopy (SEM): A Guide. AZoM, viewed 15 January 2022, https://www.azom.com/article.aspx?ArticleID=20764.
Do you have a question you’d like to ask regarding this article?
Cancel reply to comment
Mohamed Rahaman
In this interview, AZoM talks to Mohamed Rahaman, professor emeritus of materials science & engineering at Missouri University of Science and Technology, about bioceramics and their potential uses in biomedical engineering.
Dr. Duarte and Juliane Moura
AZoM speaks to Dr. Iolanda Duarte and Juliane Moura about their research that considers the presence of extremophile microbiota on photovoltaic panels.
Professor Andrea Fratalocchi
AZoM speaks with Professor Andrea Fratalocchi from KAUST about his research that focuses on a previously unrecognized aspect of coal.
Improper grease application accounts for a large amount of bearing failures. With 40% of bearings not lasting enough to deliver their engineered value, under and over lubrication are key areas to monitor. LUBExpert allows you to use the appropriate lubricant and the right time and the right place.
This is a standard rolled copper foil from JX Nippon Mining & Metals with ideal flexibility and vibration resistance properties.
Anton Paar’s XRDynamic (XRD) 500 is an automated, multipurpose powder X-ray diffractometer. It is a highly efficient and versatile XRD machine.
AZoM.com – An AZoNetwork Site
Owned and operated by AZoNetwork, © 2000-2022



