Serum Institute applies for full marketing authorisation of Covishield – Business Standard
Topics
Coronavirus | Serum Institute of India | Coronavirus Vaccine
Sohini Das | Mumbai Last Updated at January 1, 2022 00:47 IST
https://mybs.in/2Zjt5oa
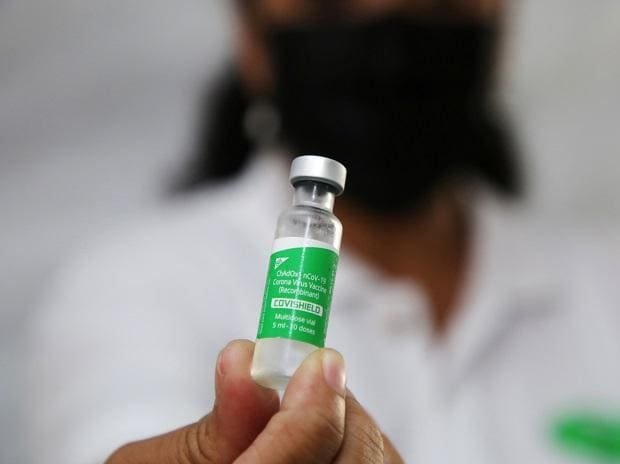
Serum Institute of India (SII) has applied for a full marketing authorization for its Covid-19 vaccine, said company CEO Adar Poonawalla on Friday.
“Supplies of the COVISHIELD vaccine in India, have exceeded 1.25 billion doses. The government of India now has enough data for full market authorisation, and therefore @SerumInstIndia has applied to the @CDSCO_INDIA_INF (DCGI) and @MoHFW_INDIA for this permission,” Poonawalla said on Twitter.document.write(““);googletag.cmd.push(function(){googletag.defineOutOfPageSlot(‘/6516239/outofpage_1x1_desktop’,’div-gpt-ad-1490771277198-0′).addService(googletag.pubads());googletag.pubads().enableSyncRendering();googletag.enableServices();});
var banHeight=$(“.article-middle-banner iframe”).height();if(banHeight<=1){$(".article-middle-banner").height(0);$(".article-middle-banner").next().next().remove();}displayConBanner=1;
Covishield received a restricted emergency use authorisation in January this year. Seurum has the capacity to make 250 million doses of Covishield monthly. The vaccine has accounted for 88 per cent of all the Covid-19 jabs given in India.
Unapproved medical product or unapproved uses of approved medical products get marketing authorisation in an emergency to diagnose, treat or prevent serious or life-threatening diseases or conditions. For full authorisation, the regulator assesses more elaborate data collected over a longer period of time.
Ashish Prasad, partner, litigation and dispute resolution practice of Economic Laws Practice (ELP), said that a full authorisation is granted to any drug or vaccine after the regulator has studied the full data submitted from phase 1, 2 and 3 trials and has deemed the product to be fit to be marketed in public. In case of an emergency use authorisation (EUA) an accelerated pathway is used where the regulator studies phase 1 and 2 data (along with interim efficacy data from phase 3 trials) to allow use of the product. Safety is not compromised in EUA.
During the Covid19 pandemic, vaccines have received EUAs from respective country regulators. In August, the USFDA granted a full authorization to Pfizer-BioNTech’s mRNA vaccine which is now marketed as Cominarty.
The research to develop a safe and effective Covid-19 vaccine has been accelerated in 2020. This has never happened before. Accelerated reviews of clinical trial data does not mean the trials have been rushed. The trials on subjects have taken their usual time, the doses being given at stipulated intervals.
The recruitment of subjects has been a faster process and the regulator has been reviewing the data from these trials from time to time. This interim reviewing of data did not happen earlier. The regulator would review the data once the trial was completed and the sponsor analysed and presented the data.
Now, the second important aspect here is that in this case, time is a factor. The regulator would need long-term immunogenicity data (whether the vaccine induces the desired immunity against a disease pathogen) from the trials. One may produce antibodies against the pathogen now, but it is important to see if the person also develops what we call memory-cell immunity. This means that if exposed to the pathogen after a long period of time (say one year) he/she still produces antibodies.
When the vaccines got EUAs, no vaccine maker had any long-term data and also real-world data. Now that the vaccines have been administered to billions of people across the globe, there is real-world data. It would capture adverse reactions (ARs) and serious adverse reactions (SARs) and based on that the regulators can now do a risk-benefit analysis, which was not possible even a few months back.
Regulators continue to review real world data even for approved vaccines. Companies themselves take up phase 4 studies of vaccines which are already in the market to gather more data on safety, efficacy and side effects.
Business Standard has always strived hard to provide up-to-date information and commentary on developments that are of interest to you and have wider political and economic implications for the country and the world. Your encouragement and constant feedback on how to improve our offering have only made our resolve and commitment to these ideals stronger. Even during these difficult times arising out of Covid-19, we continue to remain committed to keeping you informed and updated with credible news, authoritative views and incisive commentary on topical issues of relevance.
We, however, have a request.
As we battle the economic impact of the pandemic, we need your support even more, so that we can continue to offer you more quality content. Our subscription model has seen an encouraging response from many of you, who have subscribed to our online content. More subscription to our online content can only help us achieve the goals of offering you even better and more relevant content. We believe in free, fair and credible journalism. Your support through more subscriptions can help us practise the journalism to which we are committed.
Support quality journalism and subscribe to Business Standard.
Digital Editor
PREVIOUS STORY
NEXT STORY
Copyrights © 2022 Business Standard Private Ltd. All rights reserved.![]()
Upgrade To Premium Services
Business Standard is happy to inform you of the launch of “Business Standard Premium Services”
As a premium subscriber you get an across device unfettered access to a range of services which include:![]()
Premium Services
In Partnership with ![]()
Dear Guest,
Welcome to the premium services of Business Standard brought to you courtesy FIS.
Kindly visit the Manage my subscription page to discover the benefits of this programme.
Enjoy Reading!
Team Business Standard